


























cat no | io1002
ioSkeletal Myocytes
Human iPSC-derived skeletal myocytes
-
Cryopreserved human iPSC-derived cells powered by opti-ox that are ready for experiments in days
-
Ideal for the study of skeletal muscle and modelling DMD and other neuromuscular disorders
-
Functional cells contract in response to chemical and electrical stimuli in 2D and 3D microtissues

Human iPSC-derived skeletal myocytes
ioSkeletal Myocytes generated by transcription factor-driven deterministic cell programming of iPSCs using opti-ox technology
Time-lapse video capturing the rapid and homogeneous skeletal myocytes phenotype acquisition upon thawing of cryopreserved ioSkeletal Myocytes. 10 day time course.

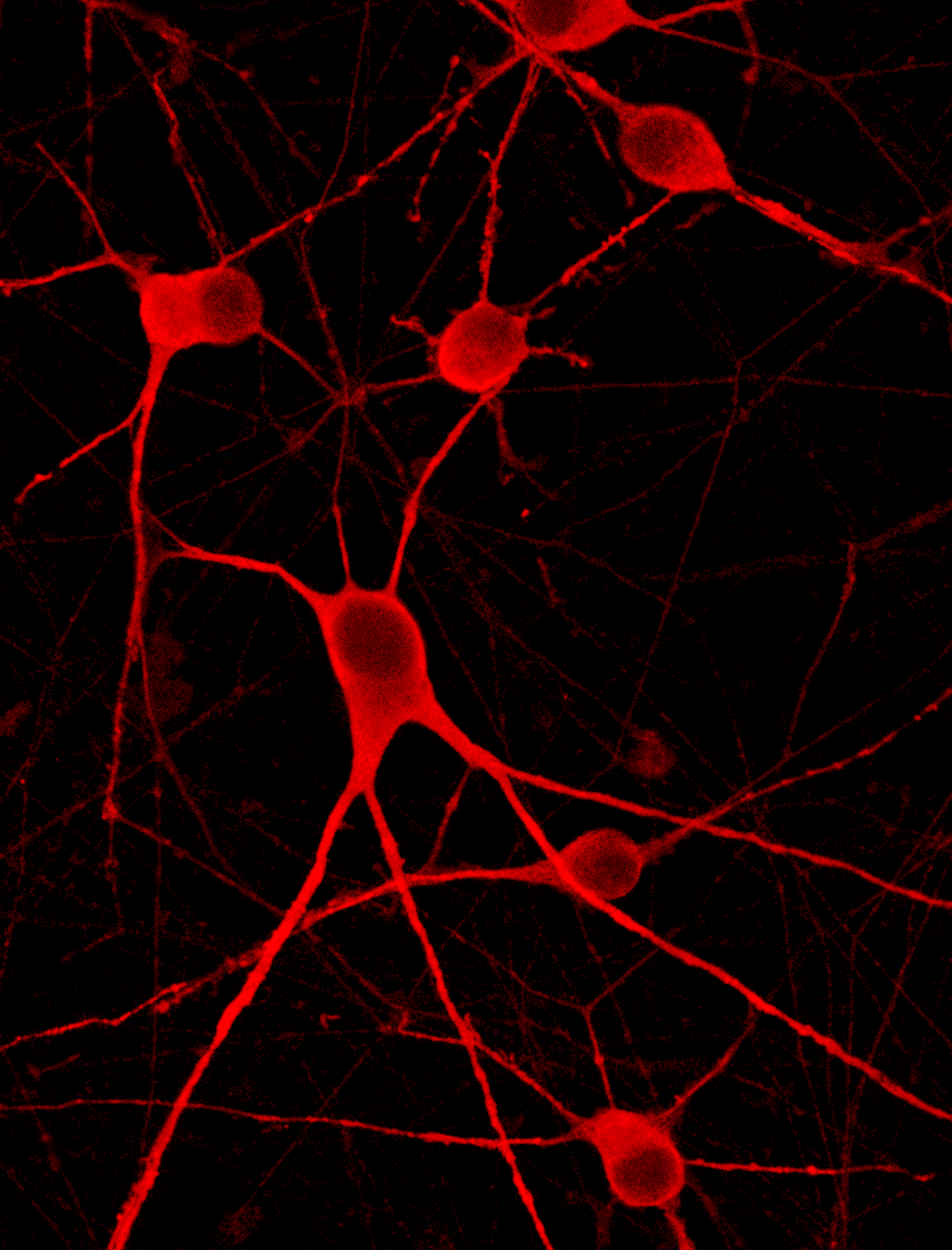
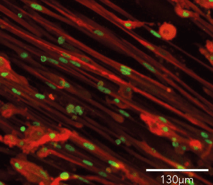
Cells demonstrate classical myocyte morphology
ioSkeletal Myocytes form elongated multinucleated myocytes over 10 days. Day 3 to 10 post-thaw; 10X magnification.

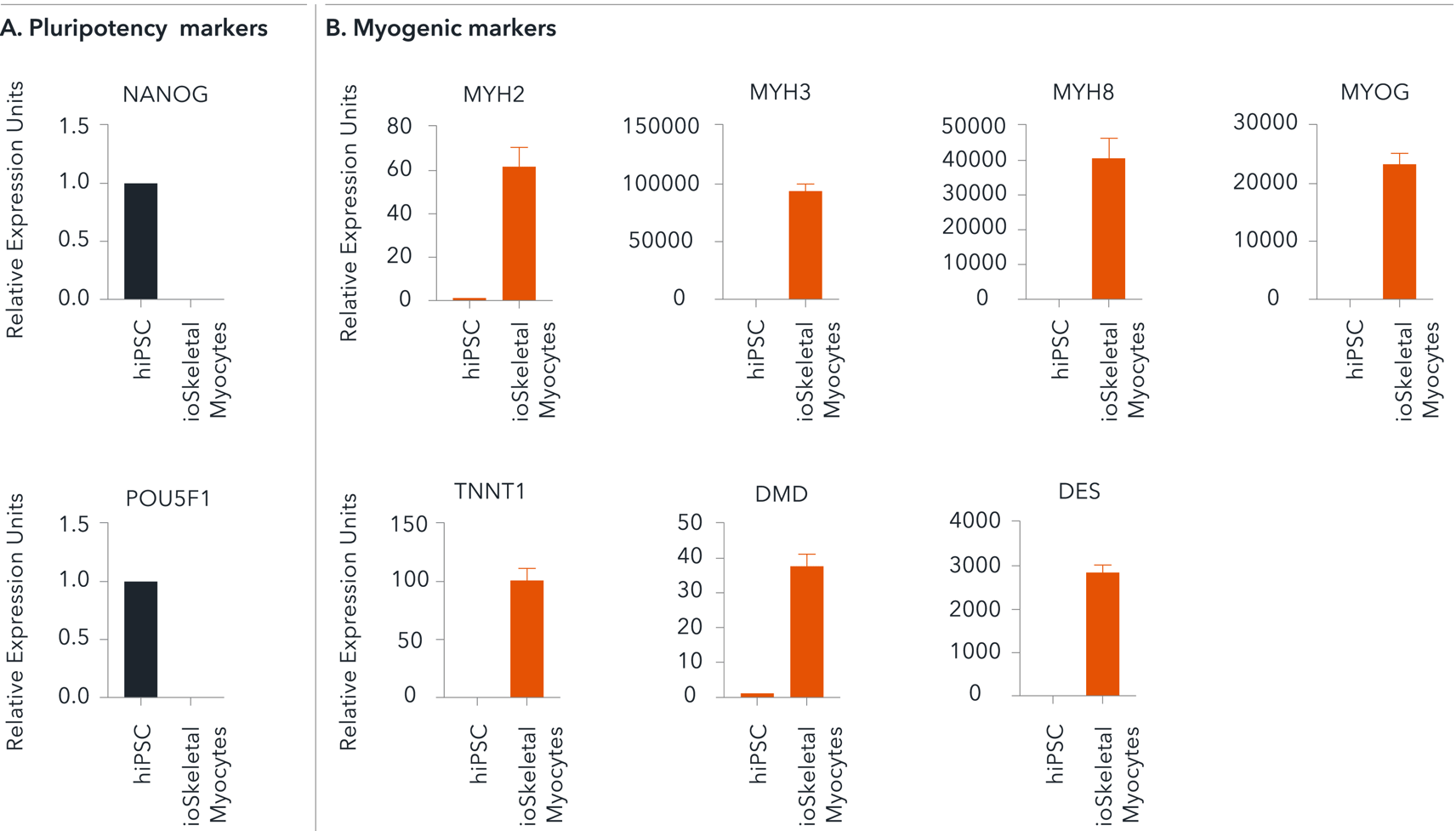
Cells demonstrate gene expression of key myogenic markers following deterministic cell programming
Following deterministic cell programming, ioSkeletal Myocytes downregulate expression of the pluripotency genes (A), while demonstrating robust expression of key myogenic markers (B). Gene expression levels were assessed by RT-qPCR (data normalised to HMBS; cDNA samples of the parental human iPSC line (hiPSC) were included as reference). Data represents day 10 post-revival samples; n=7 biological replicates.

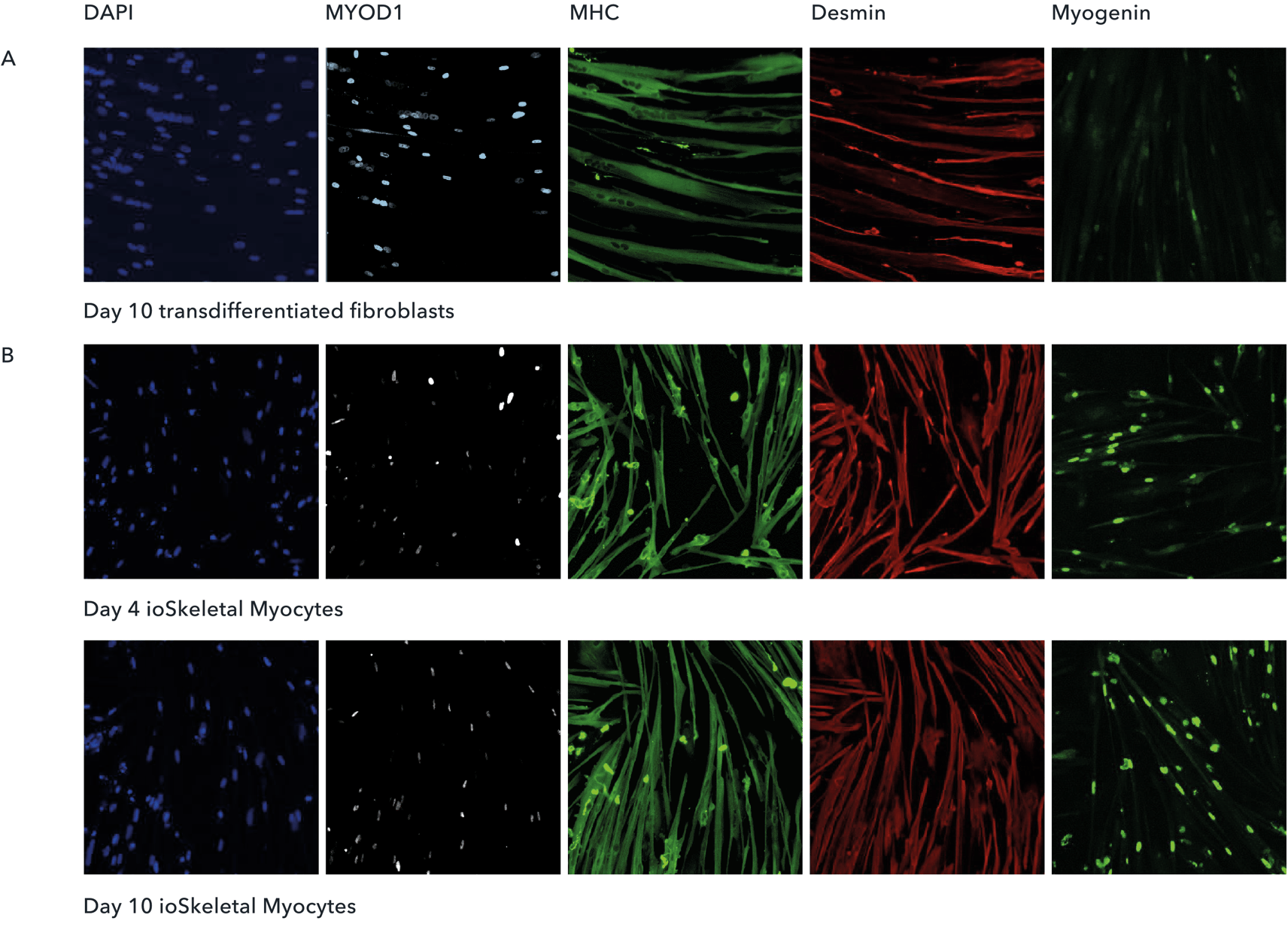
Cells are suitable for phenotypic based high-throughput screening
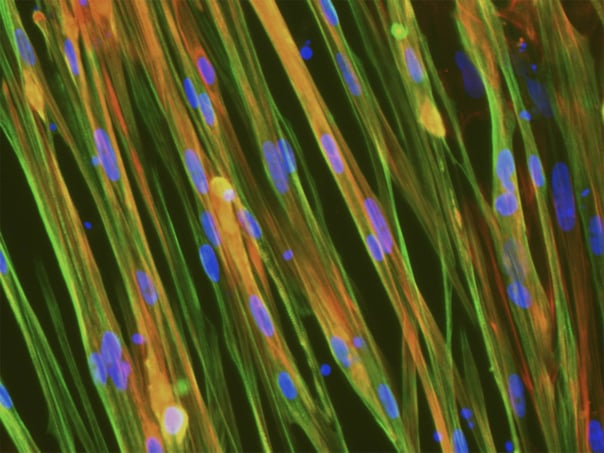
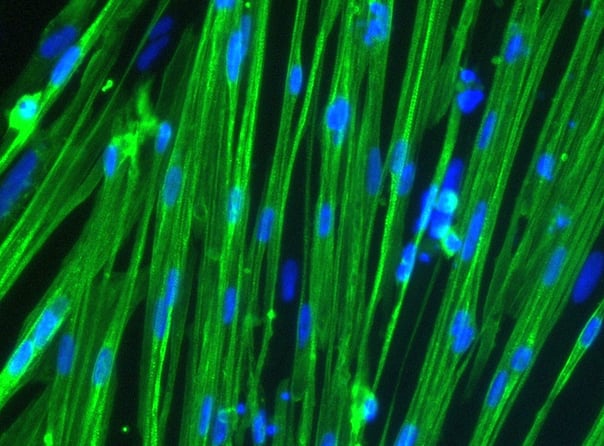
(A) Immunocytochemistry | Human fibroblasts were transduced with lentiviral vectors allowing inducible over-expression of MYOD1 to transdifferentiate them to myocytes in approximately 10 days. Transdifferentiated myotubes were stained for multiple myotube markers to assess the purity and degree of multi-nucleation. (B) Immunocytochemistry | ioSkeletal Myocytes generate myocytes within as little as 4 days post-revival with a high-degree of MHC+ cells (>80% purity), suitable for phenotypic based high throughput screens.
Shushant Jain et al, Charles River Laboratories

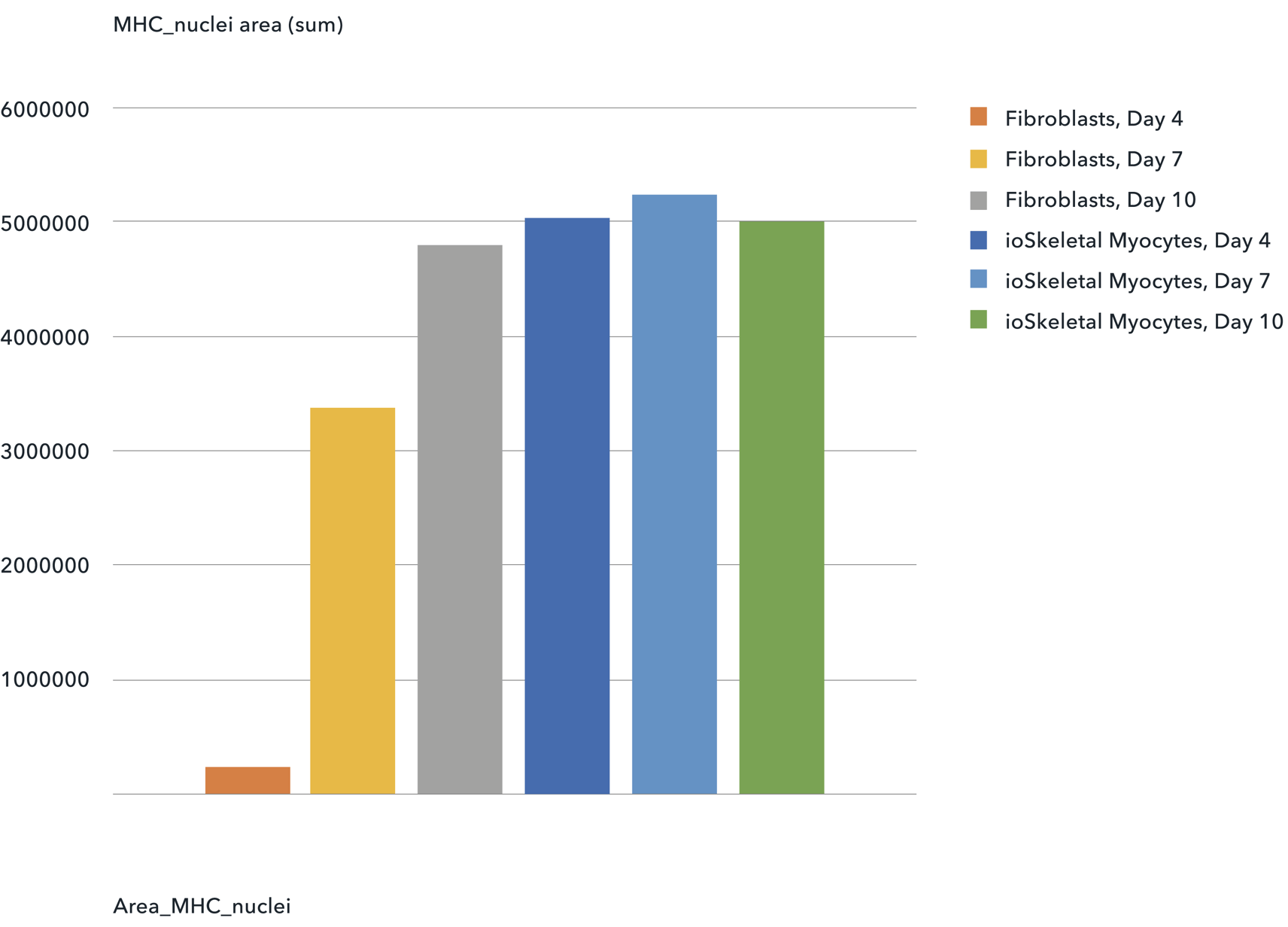
Cells are suitable for phenotypic based high-throughput screening
Myosin Heavy Chain Positive Cells | The total area of MHC positive cells generated is similar in a comparison between ioSkeletal Myocytes and transdifferentiated fibroblasts.
Shushant Jain et al, Charles River Laboratories
In vitro human muscle cells suitable for contractility assays
By day 10 post-revival, cells demonstrate a strong contractile response upon addition of acetylcholine, providing a suitable human muscle model for contractility assays. Spontaneous contraction is also observed during continuous culture (data not shown). Day 10 post-revival skeletal myocytes; 50µM acetylcholine.

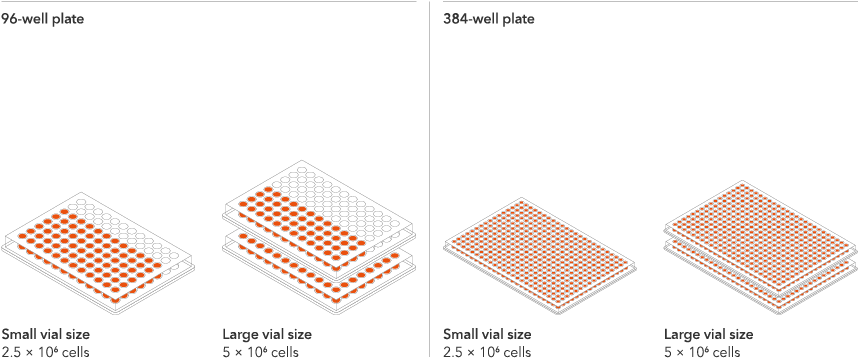
Available in two vial sizes, tailored to suit your experimental needs with minimal waste
Recommended seeding density for ioSkeletal Myocytes is 100,000 cells/cm2.
One Small vial can plate a minimum of 0.5 x 24-well plate, 0.75 x 96-well plate, or 1 x 384-well plate.
One Large vial can plate a minimum of 1 x 24-well plate, 1.5 x 96-well plates, or 2 x 384-well plates.

Contraction in response to increased extracellular potassium levels
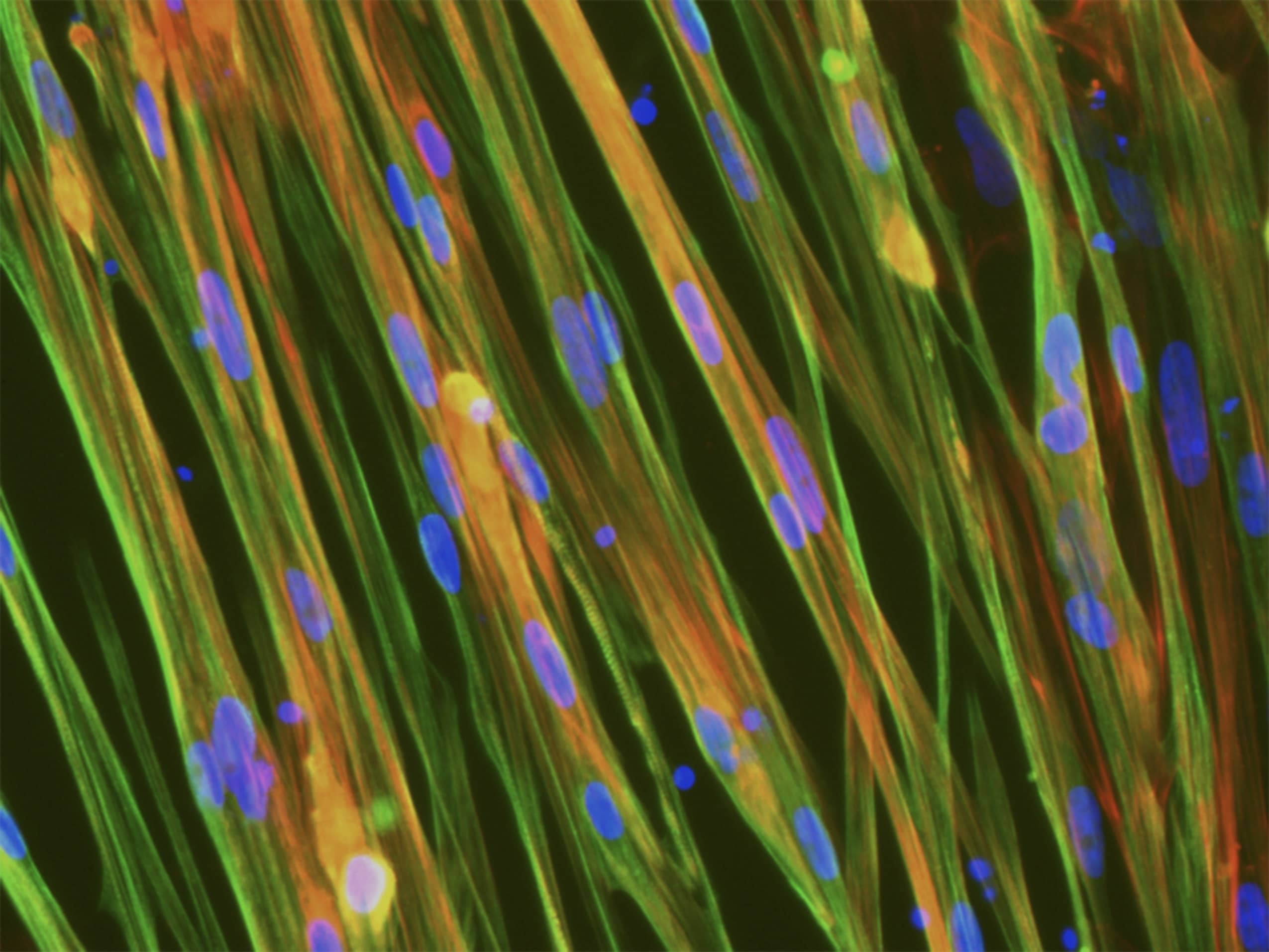
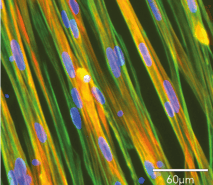
(A) Immunofluorescence staining of ioSkeletal Myocytes revealing robust expression of sarcomere structures.
Contraction is stimulated by depolarisation of the cells using potassium chloride (KCl), and the consequent increase in intracellular calcium (Ca2+) is detected using calcium binding indicator dye Indo-1 AM.
(B) Representative images of ioSkeletal Myocytes incubated with Indo-1 AM (5 µM) and 0.02% Pluronic F127; cells were excited at UV spectra (355 nm).
(C) Changes in Indo-1 AM ratio shows Ca2+ influx induced by 45 mM KCl.
Data courtesy of Gabriel E. Valdebenito and Michael R. Duchen, 2021. UCL, UK

Contraction in response to electrical stimulation
Contraction is induced by electrical stimulation and the cells release and sequester Ca2+.
The ioSkeletal Myocytes can withstand repeated electrical stimulation while maintaining their ability to regulate intracellular calcium signalling. Electrical stimulation, 2 Hz, 6 v, 2 ms.
Data courtesy of Gabriel E. Valdebenito and Michael R. Duchen, 2021. UCL, UK

Muscle bundles express muscle cell markers and show increasing maturity over time
(A) SEM image of ioSkeletal Myocytes muscle microtissues on day 14 cultured in 3D on a MUSbit microchip (Bi/ond), which includes pillars designed for anchoring muscle cell bundles; green arrow indicates muscle fibers.
(B) The cells were cultured over 14 days and expressed muscle cell markers, sarcomeric alpha actinin (SAA) and actin; a higher degree of cross-striation of SAA is seen on day 14 (yellow arrows).
Data courtesy of M. Han and M. Aarts, formerly at Bi/ond Solutions BV.

Functional 3D muscle bundles respond to electrical stimuli
(A) Twitch (black) and tetanic (red) forces are observed at day 7 for one bundle (left) and several bundles (right).
(B) Contractile force increases in one bundle (left) and several bundles (right) from day 7 to day 14, indicating muscle bundles become stronger and more mature over time.
Data courtesy of M. Han and M. Aarts, formerly at Bi/ond Solutions BV.

Functional 3D muscle bundles respond to pharmacological stimuli
(A) Contraction is inhibited when the 3D muscle cell bundle is electrically stimulated following treatment with BDM, a non-selective skeletal muscle myosin-II ATPase inhibitor.
(B) Contractility is increased when the bundle is electrically stimulated following addition of caffeine, which stimulates Ca2+ release from the sarcoplasmic reticulum.
Data courtesy of M. Han and M. Aarts, formerly at Bi/ond Solutions BV.

Statin-induced myopathy is recapitulated in 3D muscle cell bundles
ioSkeletal Myocytes were treated with a range of cerivastatin concentrations.
(A) Cerivastatin causes damage to the muscle bundle that results in reduced contraction amplitude. Bundles cultured on the MUSbit chip (Bi/ond) were treated with increasing doses of cerivastatin from day 7-14. Tetanic stimulation of 20 Hz for 1s. n=6 for DMSO, n=4 per cerivastatin concentration. Dunnett's one-way ANOVA statistical analysis: ** indicates P < 0.01, **** indicates P<0.0001.
(B) Representative images of muscle cell bundles at day 12. Cerivastatin treatment resulted in loss of myofiber organisation.
Data courtesy of M. Han and M. Aarts, formerly at Bi/ond Solutions BV.
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.







.png?width=604&name=ISSCR24-DMD-Exon44-3D-muscle-bundle%20(1).png)

/OLD%20Hero%20image%20Ex%2044%20Del.png?width=1000&height=1000&name=OLD%20Hero%20image%20Ex%2044%20Del.png)
/OLD%20Hero%20image%20Ex%2052%20Del.png?width=1276&height=1190&name=OLD%20Hero%20image%20Ex%2052%20Del.png)