17.07.2023 | Published by Science in Parliament

This article, authored by our founder and CEO Mark Kotter, featured in the Summer 2023 issue of Science in Parliament magazine.
The UK has the chance to lead the way in synbio. We must grasp it
In my previous contribution to Science in Parliament (Summer 2021) I argued that without an investment mindset containing a bolder, long-term vision for life sciences - particularly synbio - the UK risks falling behind countries such as the US. Despite increased Government priority placed upon life science since then, I want to reinforce this point.
Manufacturing human cells at a limitless scale with synbio
The timing of this article also allows me to report on a major breakthrough in the manufacturing of human cells, made possible because of our synbio approach – the idea that we can precisely engineer biology – and in this case, precisely engineer the identity of a particular human cell. Last month, bit.bio, the company I head up, presented data at the International Society for Stem Cell Research (ISSCR) Conference in Boston, showing unprecedented consistency across multiple human cell type products produced from pluripotent stem cells using a synthetic biology-based manufacturing approach called cell programming - using our precision cell programming technology opti-oxTM.
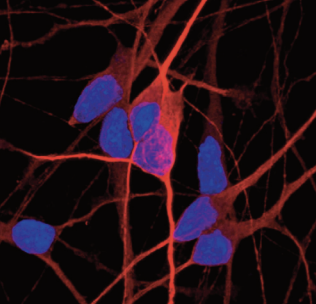
ioGlutamatergic Neurons - Immunocytochemistry staining at day 4 post revival, shows expression pan neuronal marker MAP2 (red) (counterstained with Hoechst (blue)).
bit.bio's opti-ox technology, licensed from the University of Cambridge also underpins Meatable, a scale up company that uses the same technology to manufacture cultured meat - trillions of cells on a daily basis. The technology addresses two major challenges that have held back the application of iPSC-derived cells in medical and industrial settings: consistency and scale.
Being able to manufacture human cells with consistency and scale opens the door to application in regenerative medicine, the use of cells in high throughput screening in the context of drug discovery, the generation of reproducible organoids, and the development of cell standards that help to address the lack of reproducibility that has plagued the life sciences industry. bit.bio’s unique cell products are already enabling scientific breakthroughs and applications ranging from the discovery of novel drug targets against Alzheimer’s, the development of biohybrid devices to restore paralysed limb function and outside of human cells, cultured meat.
These developments represent true disruptive innovation in stem cell biology, and the first true engineering of human biological systems. We believe that this breakthrough in cell manufacturing can be compared to the revolution in car manufacturing as pioneered by Henry Ford in terms of the integration of standard assembly components. As the first technical solution addressing the consistency and scale issue in cell manufacturing, it could have extraordinary impact.
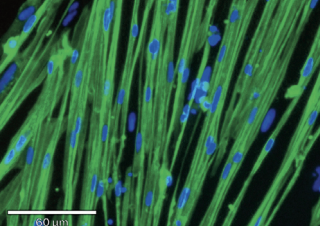
ioSkeletal Myocytes - Immunocytochemistry staining at day 10 post revival demonstrates robust expression of Titin (green) (counterstained with Dapi (blue)).
UK science is renowned for its integrity and capacity to deliver impact through its world-leading research. Yet the lack of reproducibility in research is a central challenge to the life science sector – in large part due to limited standards. In science, standards serve as universal benchmarks that facilitate accurate comparison. Achieving such standards in life sciences is much more complex as it requires standard models of biological systems like cells that are highly defined and reproducible.
Current widely used cell lines are known to have varying characteristics, making them unsuitable as a standard. bit.bio’s cell products have now reached a definition and specificity with close to no batch-to-batch variation that enables the creation of a set of standard cells. The UK has a track record in defining and exporting standards across several industries. This presents a moment for policy leaders to realise the opportunity for the UK to take a leading role in the development and strategic application of cell standards.
The US is setting the standard with bioeconomy vision
In February, I spoke at a meeting of the Parliamentary and Scientific Committee (P&SC) alongside Dr Joe Healey (CoFounder & CEO, NanoSyrinx), Dr Sara Holland (Partner, Potter Clarkson) and Fiona Mischel (Director of Human Health Content and Innovation, SynBioBeta) on the opportunities and wider landscape for the UK as a leader in synbio for human health, compared to other leading nations. Since then, the US has set out a leading ambition for its bioeconomy. The White House's report on Bold Goals for US Biotechnology and the Bioeconomy sets out its strategy as to how US Federal Departments and industry could work “to help establish R&D priorities that will be critical to advance the bioeconomy”.
The report sets out 20-year specific goals for five thematic areas of the bioeconomy including the advancement of human health and within that to “increase the manufacturing scale of cell-based therapies… and decrease the manufacturing cost of cell-based therapies 10-fold.” This work is a result of President Biden's Executive Order last September instructing federal departments and agencies to assess the potential for biotech and biomanufacturing.
Taking this collaborative approach is the correct way to develop an industry with enormous potential to drive growth across the economy and tackle some of society’s most pressing challenges. A clear statement of leadership from the US, the Executive Order sets a standard for other nations aiming for leadership status in the emergent bioeconomy. The UK needs to equal this level of ambition and coordination. Obviously, we start from a strong position considering the concentration of talent within the UK research ecosystem.
In 2022, the UK’s innovative life sciences and biotech sector secured its 5th best fundraising year with £1,785m raised by UK companies 7. Research by McKinsey in 2021 showed that between 2018 and 2020, the UK launched more new biotech start-ups than any other European country. However, the UK is falling behind the US, China, and several European countries in the number of patents granted per scientific publication; 8 per 1,000 compared to 54 in the US and 72 in China. Although the UK accounted for more than 1/3 of the total venture capital raised by European deals, the average UK deal size, at £12.4m, was lower than both the European and US averages of £18.9m and £49m respectively. As the UK’s peers put words into actions, it is imperative that we do the same in fostering direct exchange between government and industry.
Life science at the heart of science superpower ambitions
Across the UK political landscape, it is encouraging to see the increasing priority placed upon life sciences. I was pleased to see Government recognise Engineering Biology as one of its five critical technologies in the Science and Technology Framework. Government seems to be listening and answering our industry’s concerns with the planned reform of R&D tax credits. The campaign, well-led by the Bioindustry Association and supported by Anthony Browne MP (South Cambridgeshire) amongst other Parliamentarians, ensured the implementation of the new enhanced R&D tax relief rate for R&D intensive SMEs. An important correction to ensure the UK’s early-stage science base is incentivised to continue its innovation here.

Snapshot of the growing skilled workforce at bit.bio on the Babraham Campus in Cambridge.
However, the benefits of this reform will only be realised if other bottlenecks affecting life science companies are addressed, notably the availability of suitable laboratory space. Growing companies need to be able to expand but space is limited, with just 10,000 sqft of laboratory space available in Cambridge compared with 2 million sqft of demand. In the US, supply is higher with 14.6 million sqft available in Boston in 2020. On this point, the recent Spring Budget commitment to boost the supply of laboratory space is welcome.
Using regulation to drive innovation
The ongoing work by Government on regulating emerging technologies places a premium on incentivising and driving innovation. Dame Angela McLean’s recommendations from the Pro-innovation regulation of Technologies Review: Life Sciences around the focus on streamlined approvals and international partnerships for the MHRA and NICE and the creation of an Engineering Biology Regulatory Network have been accepted by Government. The regulatory approvals process is a clear area where improvements can be delivered to bring the UK closer to the speedier approval timelines for equivalent products in the US. It is reported that some approvals can take three times as long compared to those in the US, showing the scale of opportunity for UK life sciences should we be able to compete on a more level regulatory playing-field.
Investment needs to match ambition
Put simply, Government investment in life science needs to match its ambitions and the recent Life Science for Growth package is a statement of intent. Moreover, the further £3 billion of funding for British Patient Capital for the next 10 years is welcome. In return for this investment, the UK should be looking to secure its interest in the most exciting companies and technologies. The announcement in the Spring Budget of a Long-term Investment for Technology and Science (Lifts) scheme establishes new investment vehicles to crowd-in investment from institutional investors, particularly defined contribution pension funds. As the science minister observed back in 2018, the UK is a finance superpower and a science superpower, yet ironically, failed to lead on the financing of science. Now is the moment where Government could firmly harness both of these “superpowers” to secure the full economic dividend from leading UK scientific research. With a General Election approaching in the next 18 months, we are at a key moment in terms of policy consideration of biotech R&D and application to societal challenges. As acknowledged by Chloe Smith MP, Secretary of State for Science, Innovation and Technology, synbio has the potential to “revolutionise many aspects of our lives, making them longer, happier, and healthier “. The UK should look to the US and its statement of leadership in the bioeconomy on the next steps we can take to ensure a joined-up, collaborative approach to drive the synbio industry forward.
“Printed by permission of the Parliamentary & Scientific Committee”.