Our mission
Coding cells for novel cures
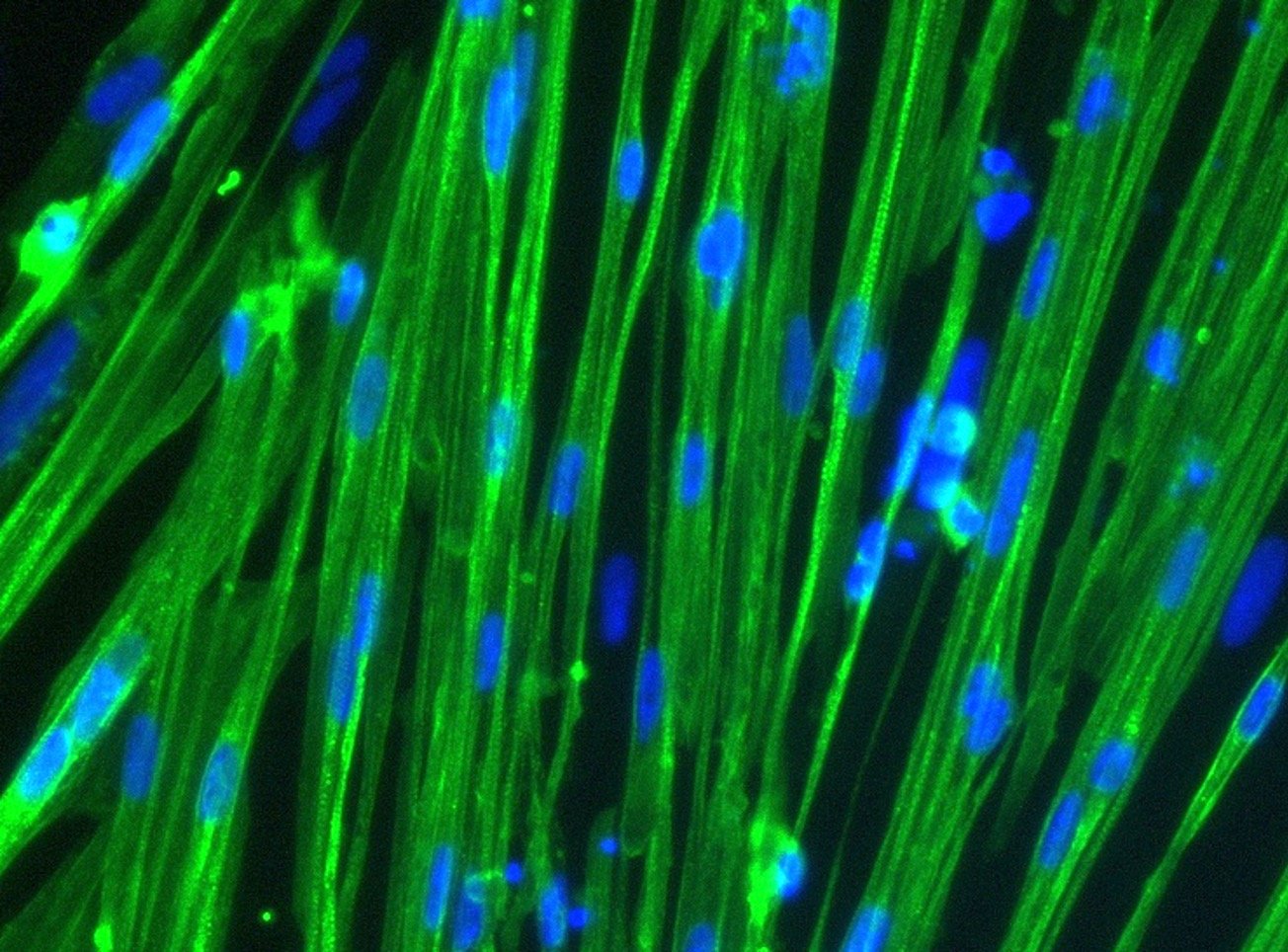



What we do
bit.bio is a synthetic biology company providing human cells for research, drug discovery and cell therapy. The company applies a patented safe harbour gene-targeting approach (opti-ox™; Pawlowski 2017) to inducibly express transcription factor combinations that reprogram human induced pluripotent stem cells (iPSCs) into highly defined and mature human cell types.
The required transcription factor combinations are discovered using high throughput screens and advanced data analysis (bit.bio discovery platform). The company is currently marketing a wide range of cells and disease models for research and drug discovery under its ioCells brand including nerve cells, immune cells and muscle cells.
The company was spun out of the University of Cambridge in 2016 and has since raised approximately $225M from leading investors, including Arch Venture, Blueyard Capital, Charles River Laboratories, Foresite Capital, M&G, Milky Way, National Resilience, and Tencent.




The company has expertise in the underpinning science of synthetic biology and stem cells, manufacturing, and clinical translation. The board includes Founder Dr Mark Kotter, Interim CEO Przemek Obloj, and Dr Richard Klausner.
Watch our Vision Video
"Transforming Medicine: Synthetic Biology for Drug Discovery and Cell Therapies"
We're driven by a bold vision—Empowering biomedical innovation and a new generation of cures through precision-programmed human cells. At bit.bio, we're harnessing the power of synthetic biology for drug discovery and groundbreaking cell therapies. Our team thrives on a simple yet powerful mission: coding cells for novel cures. It's about reimagining what's possible by programming human cells to create transformative medicines. Watch the video to see how our leadership presented the vision of bit.bio.


Przemek brings two decades of financial and investment experience focused on health, life sciences and technology across Europe and North America. Prior to joining bit.bio, he built two investment organisations, led growth and buyout transactions with combined equity investment of $5 billion and was involved in over a dozen successful exits. Most recently, he served as the Managing Partner and CIO at a Swiss family office. Prior to that, he was the Managing Director and Head of European Private Equity at PSP Investments, a C$250 billion Canadian pension manager. Earlier in his career, he worked at CVC Capital Partners, EQT, and McKinsey & Company.


Kathryn has over 25 years experience in early and late drug development across multiple indications and platforms. She was partner at Takeda Ventures, Inc. and Head of Oncology Cell Therapy Development at Takeda Pharmaceuticals where she built capabilities and oversaw a cutting edge oncology cell therapy portfolio. Prior to that, she held several roles at Sanofi Genzyme and built a two decade career at Hoffman – La Roche, Roche Molecular Systems, Eli Lilly and Syndax, contributing to the approval of 6 new cancer therapies and expansion of multiple indications.




Cornel Chiriac is an investment director with M&G Crossover Fund, focusing on late-stage investments in technology and science globally. He founded and invested for 10 years with 2050 Capital specialising in biotech and deep technology ventures. He also leads the Chicago Booth Angels Network in London and has extensive experience in strategy consulting at LEK Consulting.


Dr Richard Klausner is the founder and chief scientist at Altos Labs and a visionary leader in biotechnology. He has co-founded several leading biotechnology companies, including GRAIL, Juno Therapeutics, and Lyell Immunopharma, and has held senior executive positions at Illumina and the Bill & Melinda Gates Foundation. As former director of the U.S. National Cancer Institute (NCI), he was instrumental in advancing CAR-T therapy and shaping the nation’s cancer research agenda


Scientific advisory board
.png?width=612&height=774&name=resized%20image%20(52).png)
.png?width=612&height=774&name=resized%20image%20(52).png)




Dr. Katy Rezvani is a Professor of Stem Cell Transplantation & Cellular Therapy at The University of Texas MD Anderson Cancer Center, where she serves as the Sally Cooper Murray Chair in Cancer Research, chief of the section for cellular therapy, director of translational research, and medical director of the GMP Facility. She also serves as executive director of MD Anderson's Adoptive Cell Therapy Platform. She leads a research lab with a focus on NK cell biology and developing novel NK cell engineering strategies for cancer, with the aim of translating these discoveries to the clinic. Dr. Rezvani completed her medical training at University College London, England and her PhD at Imperial College London. She completed her training in immunology and transplantation biology at the National Institutes of Health, Bethesda, MD. In addition, she has co-authored over 200 peer-reviewed publications and has received multiple prizes and awards.




Independent ethics and sustainability board


After completing her medical studies in Rome and a post-doctoral fellowship focused on cellular immunology at the National Cancer Institute in Bethesda (USA), Dr Alteri spent 7 years in drug discovery and 5 years in clinical safety at Ciba-Geigy/Novartis in Basel. She was involved in major projects that yielded important drugs such as Gleevec for myeloid leukaemia, Coartem for malaria and Reyataz for HIV-AIDS. In 1998 she joined Serono in Geneva where she led the Pharmacovigilance and Risk Management team. At Serono, Dr Alteri was instrumental in establishing the company’s pharmacovigilance processes, as well as implementing the entire portfolio’s risk management strategy.
In 2012 she joined the European Medicines Agency, where she was Head of the Safety and Efficacy Sector for one year, being later promoted to Head of the Human Medicines Evaluation Division, and then to Head of the Human Medicines R&D Support Division.
She was member of the EMA Executive Board from 2013 until she retired in 2020.
In this roles, Dr Alteri was part of many high impact EMA’s initiatives, from the transparency of clinical trial data to the development of regulatory science strategy to 2025.
Dr Alteri has been consulting for the Swiss regulatory agency (Swissmedic), and is currently member of the Scientific Committee and faculty of the Master Course in Drug R&D of the University of Geneva. Dr Alteri regularly gives lectures on various aspects of R&D at the Universities of Rome, Cattolica and LUISS, and at the European Course in Pharmaceutical Medicine at the University of Basel. Moreover, she is member of the CIOMS Working Group XIII (Real World Data/Real World Evidence), and the CIOMS. Working Group Education. Most recently, she has been appointed as Core Member of the Proposal Review Committee of Unitaid for a 3-year period.


Marie-Claire is a pioneering international expert in sustainability, law and public policy innovation. An award-winning expert jurist, executive and law professor specialised in corporate social responsibility, climate law and governance, sustainable trade and ethical investment rules, she is Visiting Chair of Sustainable Development Law and Policy at the University of Cambridge, and also serves as Senior Director of the global Centre for International Sustainable Development Law (CISDL). Having assisted in drafting the Paris Agreement, she has published over 24 books and 160 papers with world-class presses, edited five law journals and a Cambridge University Press book series, advised the United Nations and key countries, and helps purpose-led companies to advance the UN Sustainable Development Goals.


Jeff Skopek is an Associate Professor of Law at the University of Cambridge and Deputy Director of its Centre for Law, Medicine and Life Sciences. His research explores the normative and conceptual foundations of health law, focusing in particular on controversies about what harms and benefits count. Before coming to Cambridge, he was an Academic Fellow at Harvard Law School’s Petrie-Flom Centre for Health Law Policy, Biotechnology, and Bioethics. Prior to that, he served as a law clerk to Chief Judge Lynch of the United States Court of Appeals for the First Circuit. He holds an A.B. in History from Stanford University, a Ph.D. in the History and Philosophy of Science from the University of Cambridge, and a J.D. from Harvard Law School
Our values
"At bit.bio, we are passionate about engineering human cells that will enable the medicine of the future."
Mark Kotter | MD, PhD, Founder
