Producing 3D Neuronal Microtissues for Preclinical Drug Screening using ioGlutamatergic Neurons
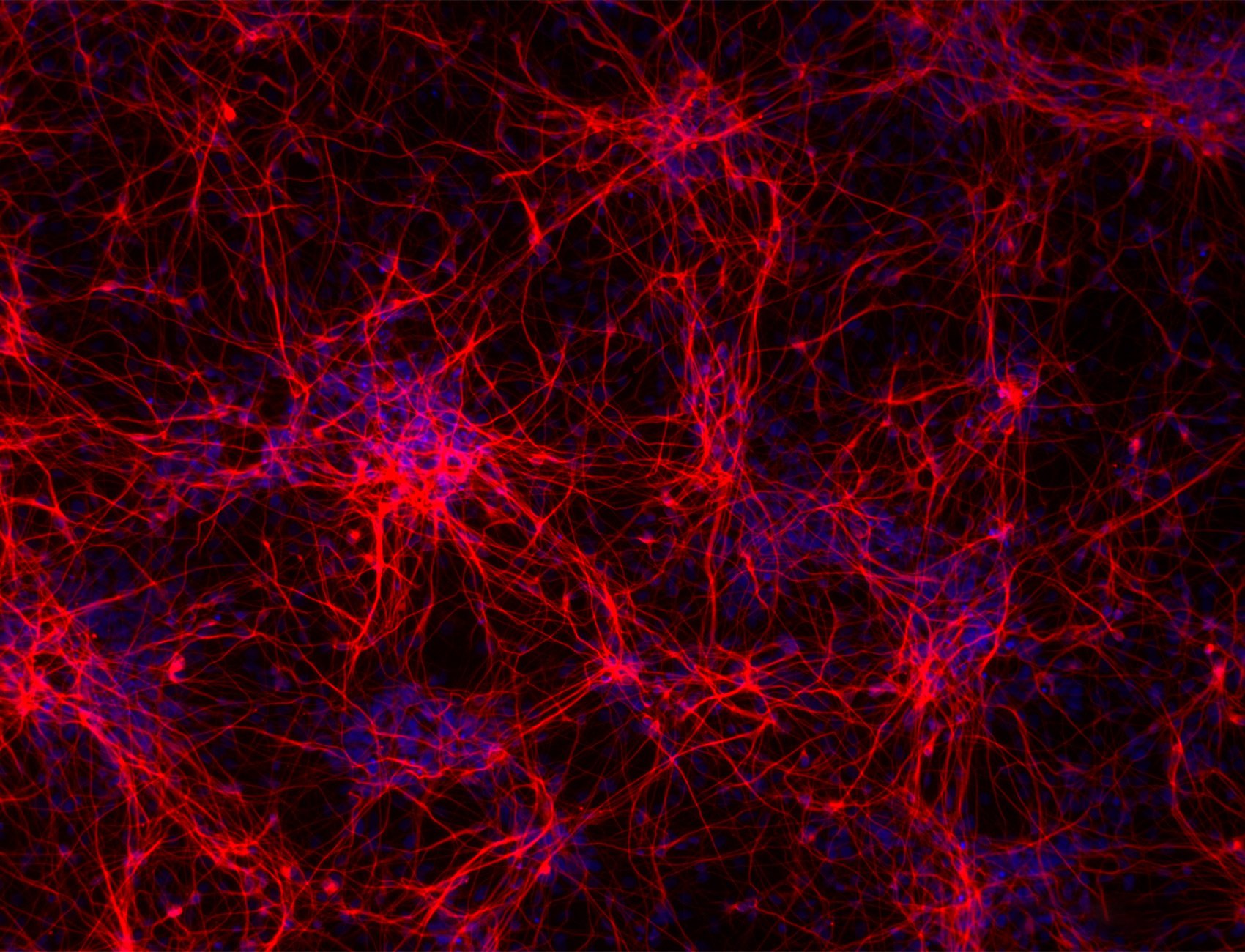
3D cell culture models using human induced pluripotent stem cells (iPSCs) have emerged as a promising, human relevant model system for the study of central nervous system (CNS) disorders. Unlike two-dimensional cell culture, 3D cultures achieve physiological relevance by culturing cells in a 3D environment with many of the factors that influence cell behavior in vivo. However, the complexity of iPSC differentiation has hindered adoption of these models for high-throughput drug screening, leaving researchers to rely on 2D models with poor predictive validity. ioCells™ from bit.bio, generated through opti-ox™ technology, provide a solution by using deterministic cell programming to consistently generate mature human neuronal cells at scale.
Herein, we delve into a recent study using the RASTRUM™ 3D cell culture platform from Inventia Life Science to generate neuronal microtissues composed of ioGlutamatergic neurons and iPSC-derived astrocytes. The microtissues are formed within a composite hydrogel scaffold mimicking key CNS microenvironmental features. These 3D co-cultures, formed in both 96- and 384-well plate formats, exhibited mature neuronal phenotypes with characteristic neurite outgrowths. Such a model offers a robust and scalable system for medium- to high-throughput drug discovery assays, thereby enabling more productive preclinical drug development.
In this application note, you will discover:
- How ioCells enable 3D co-culture of mature human neurons
- How neuronal microtissues can be built en masse using ioCells and the RASTRUM 3D cell culture platform
- Characteristics of human iPSC-derived neuronal microtissues that may be of value in medium- to high-throughput drug screening.
2024
bit.bio
Inventia