14.10.2019 | Published by Medium
14.10.2019 | Published by Medium

Over the past decades we have made tremendous progress in biomedical research. Scientists are now able to turn individual genes off or on, and edit them with CRISPR; soon we will be able to record the activity of all genes in a single cell. Therapies have progressed from small molecules to biologics; the first CAR-T cell therapies provide a glimpse of a future where cancer could be eradicated.
However, today cell therapies are expensive and not scalable, and the development of new drugs has not kept up with the progress in biology. The majority of clinical trials fail because the drugs turn out to be either toxic or they do not work in human diseases. Human biology often proves to be distinct from animal models and cell lines used for drug development. Given the lack of reliable human cells, pharmaceutical companies continue to depend on these models. To bridge the gap, a scalable source of consistent human cells is needed.
Widespread access to human cells will enable a new generation of medicine. But human cells are difficult to source . It is difficult to acquire sufficient numbers of cells from a individual, and some cell types are altogether impossible to obtain. The alternative, creating cells from stem cells, has been fraught with problems, such as inconsistency and long and complex protocols that are difficult to reproduce and scale.
(These same problems prevent the use of cells for other large scale applications, such as biomanufacturing meat or lab grown leather.)
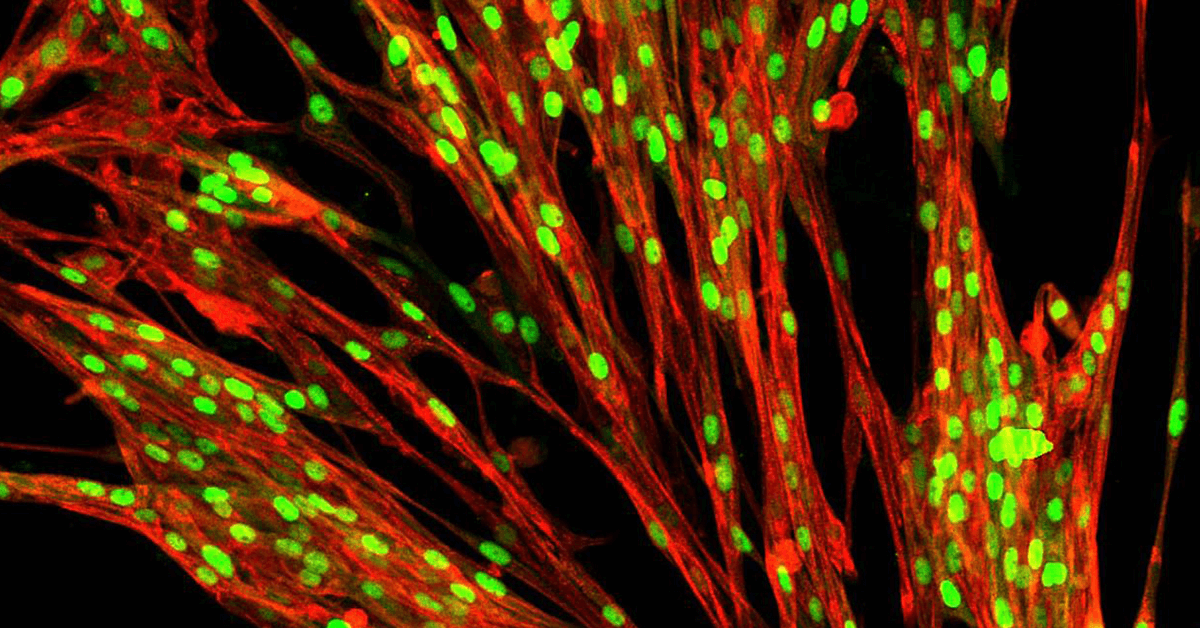
Enter cellular reprogramming. Cell reprogramming involves activating a new cell type program, skipping the usual intermediate steps that occur during development. It can be used to generate real stem cells from a blood draw or skin sample from any individual. This won Yamanaka a Nobel prize in 2012 and resolved the ethical issues surrounding the use of stem cells.
Reprogramming can also be used to convert stem cells back into any desired cell type, be it a brain, blood, or liver cell.
However, reprogramming has traditionally been inefficient with low cell yields. To overcome this hurdle, over the past five years, my research lab at The University of Cambridge has developed a gene engineering approach, opti-ox (optimised inducible over-expression). Applied to cellular reprogramming it enables precise reprogramming of entire cultures of stem cells into any desired cell type. By overcoming this bottleneck of traditional stem cell approaches, we can unlock their full potential.
Today we are excited to announce the launch of Bit Bio. Bit Bio represents the two fields ”coding and biology” that are core to our company. Bits are building blocks of code, just as cells are building blocks of life. Together, they illustrate what we do: precise reprogramming of human stem cells. Bit Bio is solely dedicated to producing functional human cells for research, drug discovery, and cell therapy.
Our moonshot goal is to develop a scalable technology platform capable of producing consistent batches of every human cell. Over the past 18 months, we have created a high throughput research platform to identify transcription factor combinations that make up cell type programs. In the next 18 months, we will leverage it with advanced deep learning algorithms to accelerate the discovery of every single cell type of the human body. Combining individual sub programs will enable us to produce cells with enhanced functions for therapeutic application. We will validate our cells and make them available for research, drug discovery, and cell therapy by working together with best in class institutions and industrial partners.
To achieve our goals, we have assembled a team of pioneers in the field of stem cells, cellular reprogramming, and cell therapy. Roger Pedersen, scientific advisor, is one of the founders of human stem cell biology and Marius Wernig, also part of our SAB, a pioneer in cell reprogramming. Together with other members of our team they have not only been pivotal for creating the field but continue to push the envelope. Experts such as Ramy Ibrahim, a leader in Immuno-oncology, and Florian Schuster, previously involved in growing a cell therapy company, have joined Bit Bio as scientific advisor and CFO/COO, respectively. Our commercial team is led by serial biotech entrepreneur and CBO, Paul Morrill.
When Intel launched, the idea of a widespread use of computers was a remote fantasy. Today, there are few areas of our lives that have not been transformed by them. Creating a standard for semiconductor chips formed the basis of our entire IT industry. What Bit Bio is doing is similar: building a platform that, if it works, can transform biomedical research: enable scientists and innovators to focus on what they do best, and leverage Bit Bio’s cell coding platform to provide the cells.
Original medium.com article